May2020/2 - “Mi KASA es su KASA”; the FDA initiative
In this blog I would like to inform you on the KASA initiative of the FDA. KASA stands for Knowledge-aided Assessment & Structured Application and this future system should help the agency in ensuring and overseeing pharmaceutical quality in an efficient, consistent and objective way.
The intent of KASA (see fig.1) is to build a knowledge-base of pharmaceutical product design, manufacturing and facilities that will allow the FDA to make risk-based decisions with regard to the regulatory oversight needed, for a new drug application or post-approval changes of an existing drug. Bottomline, KASA should capture and manage – in a standardized and retrievable manner - the information of the FDA regarding inherent product, manufacturing and facility risks and how these risks are being controlled by an applicant. For further reading see: Lawrence X. Yu et al, International Journal of Pharmaceutics (2019), available online: https://www.sciencedirect.com/science/article/pii/S2590156719300246 .
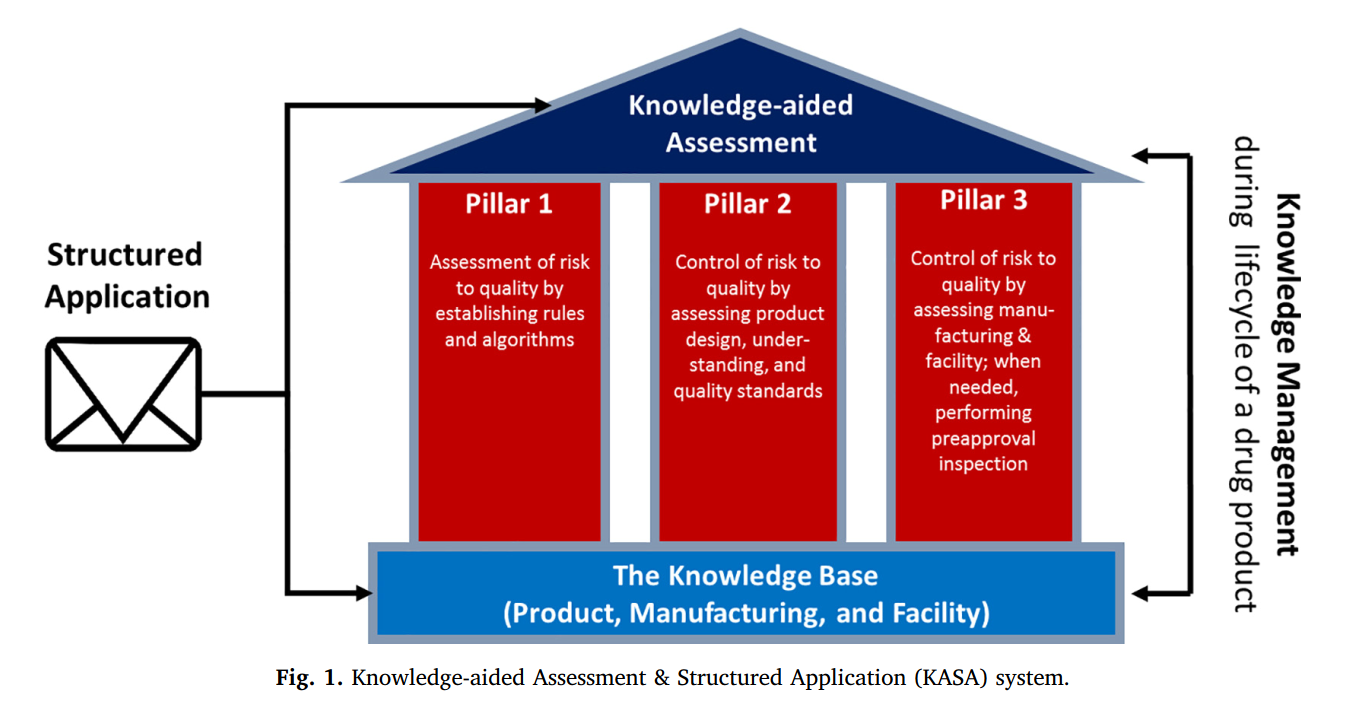
Although it is not defined when KASA will go live, this FDA initiative is another example of the importance of Quality Risk Management (QRM) in drug development and manufacturing. Lucas GxP Consultancy can provide support in the design or improvement of your QRM system or even help you in the execution of Risk Assessments (RA) as defined on your QRM plan. The RA support can be provided on site but we recently developed additional tooling for the execution of RA’s via web-meetings. The first experience with a web-hosted RA of a customer in Germany was very good!
For more information, please contact Lucas GxP consultancy on +31 6 36258808 or submit an email to hans.lucas@gxpconsultancy.com

